Have you ever tried to bake a cake without following a recipe? Chaos, right? Chemistry, in many ways, is a lot like baking. You need the right ingredients in the right proportions for a successful outcome. That’s where balancing chemical equations comes in! Just like a recipe tells you how much flour and sugar to use, a balanced chemical equation tells you the exact amount of each element needed for a chemical reaction to occur.
What Does Balancing a Chemical Equation Even Mean?
Imagine you’re building a tower with Lego blocks. You start with a pile of blocks (reactants) and end up with an awesome tower (product). But here’s the thing: you can’t create or destroy Lego blocks, you can only rearrange them!
Balancing chemical equations follows the same principle, which is based on the Law of Conservation of Mass. This law states that in a closed system, matter cannot be created or destroyed, only transformed. So, in a chemical equation, the number of atoms of each element you start with (reactants) must be the same as the number of atoms of that element you end up with (products).
How to Balance Chemical Equations: A Step-by-Step Guide
Now, let’s unlock the secret to balancing those chemical equations:
1. Write the Unbalanced Equation
Start by writing down the chemical formulas of the reactants and products. For instance, the reaction of hydrogen gas (H₂) with oxygen gas (O₂) to form water (H₂O) would be written as:
H₂ + O₂ → H₂O
2. Count the Atoms
Next, count the number of atoms of each element on both sides of the arrow. In the example above:
- Reactants: 2 hydrogen atoms, 2 oxygen atoms
- Products: 2 hydrogen atoms, 1 oxygen atom
3. Adjust the Coefficients
To balance the equation, you can only change the coefficients, which are the numbers in front of the chemical formulas. Never change the subscripts, as that would change the chemical identity of the substances!
To balance the oxygen atoms in our example, we need to put a “2” in front of the water molecule (H₂O):
H₂ + O₂ → 2H₂O
However, now we have 4 hydrogen atoms on the product side and only 2 on the reactant side. To fix this, add a “2” in front of the hydrogen molecule (H₂):
2H₂ + O₂ → 2H₂O
4. Verify Your Work
Finally, double-check that the number of atoms of each element is the same on both sides of the equation. In our balanced equation, we have:
- Reactants: 4 hydrogen atoms, 2 oxygen atoms
- Products: 4 hydrogen atoms, 2 oxygen atoms
Voilá! The equation is balanced.
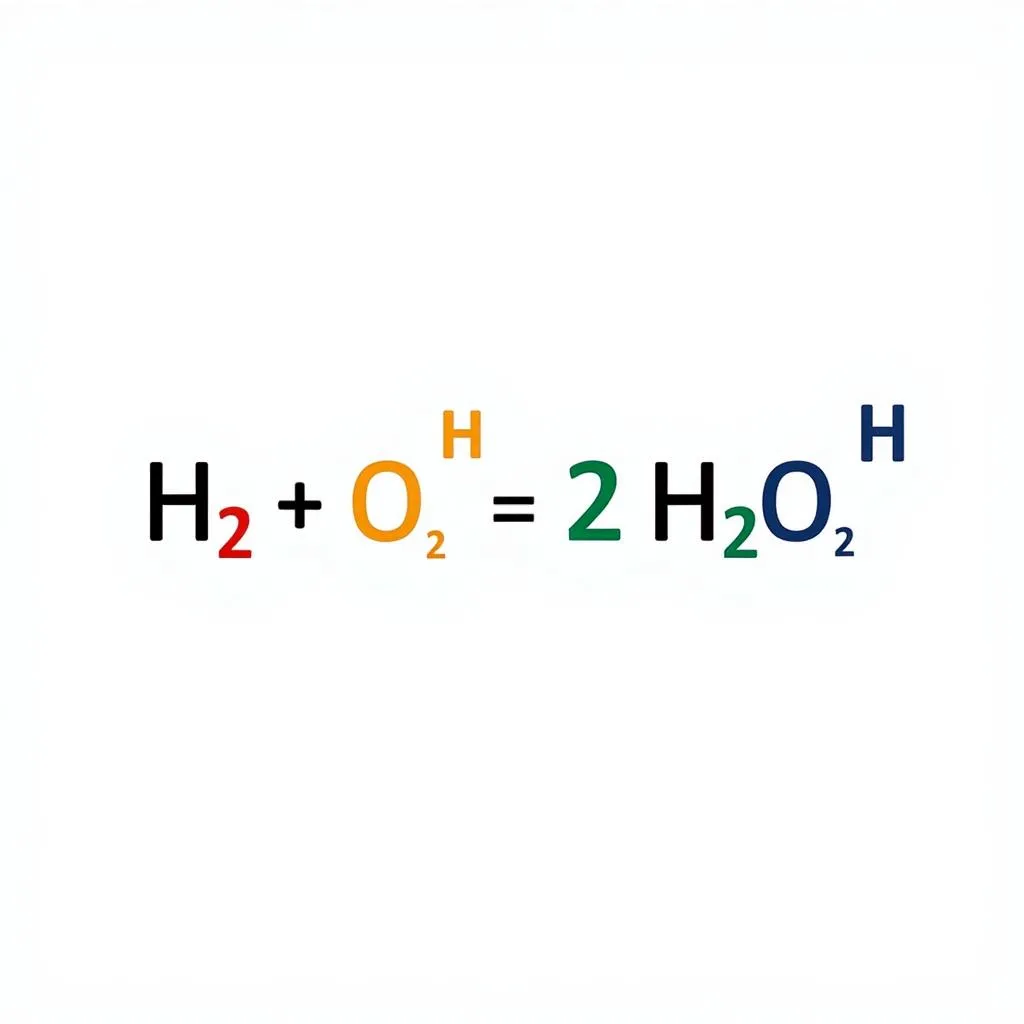 Example of a balanced chemical equation
Example of a balanced chemical equation
Balancing Equations: More Than Just Science
In Vietnamese culture, balance plays a crucial role, reflecting the philosophy of Yin and Yang. This concept emphasizes the interconnectedness and interdependence of seemingly opposite forces, striving for harmony and equilibrium. Similarly, balancing chemical equations highlights the delicate balance in the natural world, where even the smallest changes can have significant effects.
Just as a skilled chef in Hanoi’s Old Quarter balances flavors to create a harmonious dish, a chemist carefully balances equations to predict and control chemical reactions.
Need Help Navigating the World of Chemical Reactions?
Whether you’re a student grappling with chemistry concepts or just curious about the world around you, understanding how to balance chemical equations is a fundamental step. And remember, just like exploring the bustling streets of Hanoi, learning about chemistry is an adventure waiting to be discovered!
Contact TRAVELCAR at 0372960696 or [email protected]. Our friendly team is available 24/7 to assist you with your transportation needs in Hanoi and beyond, ensuring you have a smooth and enjoyable experience.

